

A New Path to GI Health
Digma Medical Ltd, is a leading medical technology company dedicated to transforming the treatment of gastroenterology pathologies starting with Barrett’s Esophagus (BE) condition, which may lead to An Esophageal Adeno Carcinoma, one of the leading deadly cancerous diseases. By focusing on this critical pre-cancerous condition, we aim to offer innovative, patient-centered solution that provides a more successful alternative to traditional methods, ultimately improving patient outcomes and their quality of life.
Digma is developing a minimally invasive therapy, Endoscopic Gastrointestinal Ablation Device (EGAD), that provides contactless, uniform, deep ablation, guided by imaging, allowing to treat the full circumference or isolated patches in a single, safe and fast procedure. The treatment targets the Esophagus mucosa tissue while protecting deeper layers like the muscularis.
Our product consists of a console and a single-use catheter that is compatible with the working channel of standard gastrointestinal endoscopes. The EGAD procedure is being developed so that it can be performed in a standard GI endoscopy suite without requiring additional imaging equipment. Our goal is to provide patients with durable improvement in Barrett's Esophagus with minimal side effects and with reduced disease reoccurrence.
The EGAD procedure is currently being evaluated in clinical studies. To learn more about our clinical program, please contact us here.


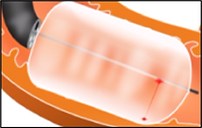
Barrett's Esophagus
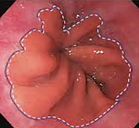
Barrett’s Esophagus Condition:
-
Caused by chronic GERD (Gastroesophageal reflux disease)
-
Often progresses silently, diagnosed too late
-
Symptoms: heartburn, regurgitation, difficulty swallowing
-
Increases risk of esophageal adenocarcinoma (EAC) by up to 125x
-
Ablation used to eliminate abnormal tissue & enable regeneration
-
High recurrence with current therapies & a few sessions per patient
-
Rising demand for effective, safe, and minimally invasive solutions
Endoscopic GI Ablation Device (EGAD) for a Uniform & Tailored ablation

-
A precision ablation; Controlled depth and energy (Map & Mark before Ablate)
-
“One and Done” – Single tool, multi-zone treatment
-
Effective ablation without a full tissue contact
-
Shorter procedure, avoid a general anesthesia
EGAD is a minimally invasive LASER-based balloon-catheter delivery:

-
Adjustable and selective tissue interaction for various anatomies.
-
Outpatient procedure in GI suite using standard endoscopy technique (No fluoroscopy).
-
Through-the-scope delivery
-
Balloon system centralizes the laser; Catheter Anchoring
-
Visible RED guide pointer. NIR as a therapeutic laser
The global Barrett's Esophagus Ablation Devices market:
-
$1.5B market by 2033 (CAGR 7.8%)
-
>12M affected in the US
-
200K+ new U.S. cases/year
-
Large unmet need and high recurrence drive demand
-
EAC accounts for 60% of Esophageal cancer, the 6th most common cancer
Leadership Team
SHLOMIT CHAPPEL-RAM, PhD, MBA
General Manager

Dr. Shlomit Chappel-Ram is an accomplished executive with over 20 years of leadership in the medical device and life sciences industries. At Digma Medical, she leads strategic operations, product development, and fundraising efforts for the company’s endoscopic laser therapy platform. Prior to Digma, she held senior roles at Nano Dimension, Edwards Lifesciences, and Medinol, driving innovation from early-stage development through commercialization. Dr. Chappel-Ram holds a PhD in Chemistry (Summa Cum Laude) and an MBA in Management, and actively contributes to healthcare innovation initiatives in Israel and abroad.
ILAN BEN-OREN
Co-founder and CTO

Ilan brings more than 25 years of experience in R&D and management in the medical device industry. In addition to co-founding Digma, he served as founder and CTO of Eximo Medical (acquired by AngioDynamics), was the CTO of Exalenz Bioscience Ltd., (acquired by Meridian BioScience) and was CTO at Oridion Medical (acquired by Covidien). Ilan is the author of more than 60 patents and patent applications in the field of medical technologies and electro-optics. Mr. Ben-Oren holds a master’s degree in applied physics and the science of materials summa cum laude from the Hebrew University of Jerusalem.
Dr. IRIT YANIV, MD
Chairwoman of the BOD, Co-Founder

Dr. Irit Yaniv is the Founding Partner and CEO of Almeda Ventures,
and Managing Partner at Accelmed Ventures II, with over 25 years of experience in the venture capital, pharmaceutical and biotech industries. As a senior manager, skilled physician, and experienced board member, she possesses a highly developed combination of leadership, decision making and business skills. She holds an MD from Ben-Gurion University and an MBA from Tel Aviv University.
Prof. Ohad Etzion is a director of the Department of Gastroenterology and Liver Diseases at Soroka University Medical Center. Vast experience in leading multicenter/ multinational clinical studies. Consultant to multiple companies in the bio pharmaceutical and medical device industries.
Prof. OHAD ETZION, MD

Scientific Advisory Board Member
